World leader in particle accelerator technology
IBA is a unique scientific company characterized by a deep human connection that is illustrated by :
![]()
Image
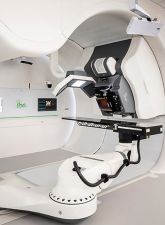
Proton Therapy
Image

Dosimetry & Quality Assurance
Image

RadioPharma Solutions
Image

Industrial Solutions
IBA designs, produces and markets innovative solutions for the diagnosis and treatment of cancer and other serious illnesses, and for industrial applications such as the sterilization of medical devices.
